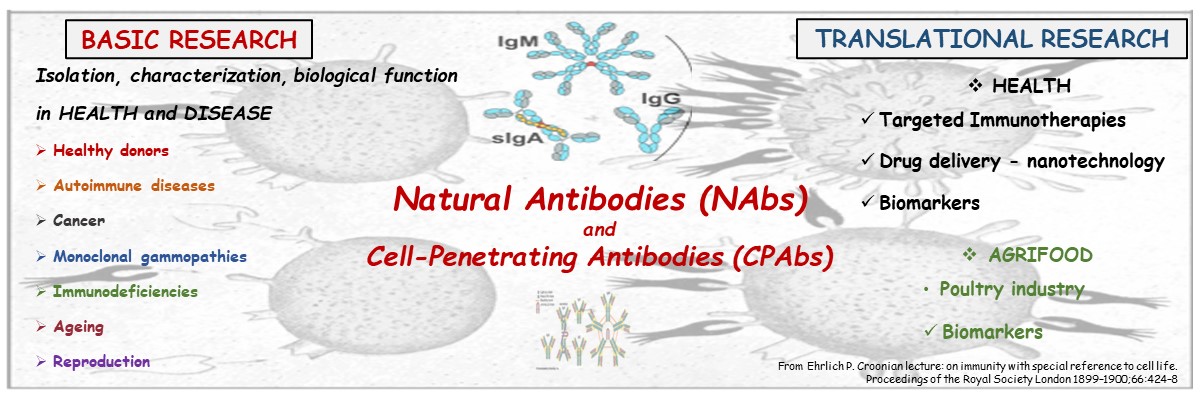

The main research activity of the Laboratory of Immunology (Lymberi group) is the study and utilization of natural or naturally-occurring autoantibodies (NAbs) in health and agrifood, as biomarkers and therapeutic tools. Emphasis is placed on the recently identified ability of NAbs to penetrate living cells with the aim of using them as carriers for the intracellular transport of pharmaceutical agents unable to pass through the cellular membrane, for the targeted therapy of diseases such as cancer.
NΑbs are the group of antibodies present in healthy organisms in the absence of intentional immunization. They are found in the bloodstream (>90% of circulating Ig of all classes) as well as in other biological fluids and recognize several antigensX of different composition, self and non-selfX exogenousX antigens, a characteristic defined as polyreactivity. This property is closely related to the remarkable biological roles of NAbs, such as the removal of cellular and molecular waste, the resistance against infections, cancer surveillance and immunoregulation and homeostasis. The immunoregulatory role of NAbs appoints them as valuable diagnostic, prognostic and therapeutic agents. NAbs have been successfully used in therapy, mainly through polyclonal intravenous immunoglobulin preparation (IVIg) -produced from an immense number of healthy donors-, while monoclonal NAbs have been used in Multiple Sclerosis.
As NAbs can be exploited as biomarkers in agrifood, our laboratory has undertaken the research and development of new immunological and biochemical quality markers in free-range chickens.
The most recently identified activity of NAbs is their ability to penetrate living cells. Our laboratory isolated, for the first time worldwide, natural human polyclonal and monoclonal CPAbs from healthy donors. Basic research projects are focused on the biological and functional characterization of natural human monoclonal CPAbs. At translational research level, the laboratory studies their use in the intracellular transport –both in the cytoplasm and the nucleus- of pharmaceutical agents unable to pass through the cellular membrane, for the targeted therapy of diseases such as cancer.
In response to the demands and priorities imposed due to the COVID-19 pandemic, we have been working since the beginning of the crisis towards developing highly specific and sensitive enzyme immunoassays for the detection of antibodies against SARS-CoV2 antigens (Nucleocapsid, Spike, S1, RBD) for monitoring of the serological immune response in COVID-19 patients and following vaccination in the Greek population (in collaboration with researchers of the Hellenic Pasteur Institute and the Institut Pasteur Paris).
In addition, our research activities include the study of the autoimmune disease myasthenia gravis (MG) and specifically the development of an antigen-specific therapy based on restoring immunological tolerance towards the targeted autoantigens. (Lazaridis group)
The laboratory is also actively involved in students’ education through the supervision of graduate, MSc and PhD theses as well as the organization of, both HPI and national, seminars regarding Immunology and its biomedical applications.
1. Cell penetrating polyreactive human autoantibodies (pcPAbs) as innovative therapeutic bio-tools.
Νowadays, it is established that there are autoantibodies able to penetrate living cells (Cell-Penetrating Antibodies, CPAbs) and locate in the nucleus and/or the cytoplasm. CPAbs have been found in patients with autoimmune diseases such as systemic lupus erythematosus and lupus-prone mice, whilst our laboratory isolated, for the first time worldwide, natural human polyclonal and monoclonal CPAbs from healthy donors. More specifically, we isolated, from three therapeutic formulations of intravenous immunoglobulin IVIg, natural human polyclonal pcPAbs and further characterized their cell-penetrating ability and intracellular effect. Based on our published data, the laboratory proceeded with the development of human monoclonal pcPAbs that penetrate the cytoplasm of living cells and focused on the study of their multiple intracellular roles. Those antibodies are endowed with distinct biological properties such as induction of apoptosis in cancer cells as well as targeting of molecules with nodal roles in pathologies such as cancer and Alzheimer’s disease. Furthermore, as those antibodies also possess the ability of intracellular transport of diverse biomolecules (e.g. peroxidase, FITC, polystyrene particles) the Laboratory is engaged in the combined use of nanotechnology (drug loaded nanoparticles) with carrier CPAbs with antineoplastic activity, aiming at improved targeted therapies.

2. Free range pastured Poultry biochemical Indicators, Novel and Dynamic, to certify Outdoor rearing Systems
This project participants are The Biochemistry Laboratory of the University of Ioannina, the Agricultural Poultry Association of Ioannina (APSI «PINDOS») and the immunology Laboratory of HPI. Those entities carry out together a large industrial research whose goals are:
a) the development of methods, mechanisms and tools of verification of the authenticity of free-range poultry husbandry and consumer protection from fraud and adulteration of high value added as well as
b) the establishment of an innovative dietary method of poultry farming, unique in a national level, leading to an increase of the internal value added and a production of high quality end products with exportation potential.
The HPI Immunology Laboratory has undertaken the research and development of new immunological and biochemical quality markers in free-range chickens, through the study of 1. Natural Autoantibody levels of IgM and IgY(G) class to a series of self and non-self exogenous antigens, and 2. Chicken-Specific Troponin-T peptides.
The emergence and improvement of the special characteristics of Greek products of primary production by applying innovative production processes is a main step towards fulfilling the Regional development policy in the Agri-food industry, as expressed in the Smart Specialization priorities (RIS3) of the Region of Epirus.

3. SARS-CoV-2 Reserach (2020-2022): Monitoring of the serological immune response upon COVID-19 mRNA Vaccination in Greece through the use of in-house ELISAs
Hellenic Pasteur Institute (Decision of the Administration Council, Reference Number: 14365 / 28.12.2020) Project: Development of Serological immunoassays to get insight into the anti-SARS-CoV-2 immune response in Greece. In collaboration with the HPI Labs: Molecular Biology & Immunobiotechnology and Public Health-Diagnostics, and the Institut Pasteur Paris (provided us the respective plasmids for SARS-CoV-2 antigen expression at the HPI Biotechnology Unit). Project Coordinator: Dr P. LYMBERI)
Serology testing of SARS-CoV-2 is increasingly being used during the current pandemic of COVID-19, not only in infected but also in vaccinated individuals. Characterizing these antibody-based SARS-CoV-2 assays helps to understand the disease and the vaccination effectiveness and provides scientific basis for deciding how to best use them. This project is focused on the development of in-house highly specific and sensitive diagnostic antibody-based enzyme immunoassays (ELISAs) by using the recombinant expressed immunogenic proteins of the virus Spike (its trimeric native form) and Nucleocapsid N protein, as well as specific and potent Spike’s fragments, such as S1 and Receptor Binding Domain.
In December 2020, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the Pfizer-BioNTech (BNT162b2) SARS-CoV-2 vaccine, an mRNA-based vaccine that encodes full-length viral S ectodomain (SARS-CoV-2 Spike protein). The vaccination was initially prioritized for individuals at high risk of SARS-CoV-2 exposure, such as the healthcare workers (HCWs) and for those at high risk of severe COVID-19, such as elderly and residents of assisted living facilities. We have analyzed a cohort of 146 Greek HCWs of a Greek hospital regarding the overall anti-Spike and the targeted anti-Receptor Binding Domain IgG and IgA antibody responses after vaccination with BNT162b2 mRNA vaccine at seven distinct time points, in a period of 9 months. The effect of the 3rd vaccine dose was also assessed.
Development of an antigen-specific therapy for Myasthenia gravis
Myasthenia gravis (MG) is an antibody-mediated autoimmune disease affecting the neuromuscular junction, causing muscle weakness and fatigability. In the majority of patients (85-90%) it is caused by autoantibodies targeting the muscle acetylcholine receptor (AChR), thus impeding neuromuscular signal transmission. Although the B cell-produced autoantibodies are the pathogenic agents in MG, T cells play a central and indispensable role in disease development, promoting the activity of autoreactive B cells.
The major MG autoantigen, the muscle AChR, is a pentameric transmembrane protein composed of four homologous subunits with the stoichiometry 2α1-β1-ε-δ (or 2α1-β1-γ-δ for embryonic receptors). Interestingly, most of the autoantibodies target the α1 subunit. Furthermore, a number of T cell epitopes have also been identified within the α1 subunit.
Our team (together with the Molecular Neurobiology and Immunology laboratory of the HPI) has developed and characterized in great detail an experimental autoimmune MG (EAMG) model in rats, using immunization with domains of the human AChR subunits, which results in highly reproducible symptom emergence and progression. Due to the use of human proteins for disease induction, this model is ideal for the study of antigen-specific MG immunotherapies.
We are working towards the development of a therapeutic strategy aiming to restore the lost tolerance towards the muscle AChR. The treatment rational is based on chronic administration of the autoantigen or its peptides under specific tolerance-inducing conditions. Recent findings in in vivo studies have shown a significant reduction of symptoms in treated animals compared to untreated or mock-treated ones. Our main efforts currently are focused on elucidating the precise mechanisms and cell populations involved in this response, which will not only increase our understanding and facilitate progression towards clinical application, but also potentially allow the development of even more targeted approaches.
NATIONAL PROGRAMS
2019-2021
National Strategic Reference Framework (NSRF), Human Resources Development, Education and Lifelong Learning, in the context of “Support to researchers with emphasis to young researchers-Round B’” Call, Proposal no: 1091, Research Field: Medical & Biological Sciences, Title:” Study of human, natural, monoclonal antibodies endowed with cell-penetrating ability as a model of targeted and combinatorial anti-cancer therapy”. Project Coordinator: Dr P. LYMBERI/ Amount of grant: 45.546,00 €
2018-2020
Operational Programme Competitiveness, Entrepreneurship and Innovation 2014-2020 (EPAnEK); sectoral operational programme of the Partnership and Cooperation Agreement (the new NSRF) for the period 2014-2020, approved on 18/12/2014 by the European Commission. MIS 5055808 (Proposal no: T2ΕΔΚ-01371, 18-2-2020), AREA I. R&D of Companies /PRIORITY AREA 5: Health and Medicines/AREA 5.7 Identification and confirmation of new therapeutic means, goals and biomarkers for the development of new methods/ PRIORITY 5.7.1 Individual analysis of human genomes and development of new methods. Titre: “Development and Commercialization of a microbiome-based prediction test for in vitro fertilization.”. Acronym: Mi-IVF. Collaborating Private Companies: 1. AKESSO, 2. CEMIA S.A. Subcontractors: 1. Pharmacogenomics Laboratory, 4th Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens. 2. Immunology Laboratory, Dept of Immunology, Hellenic Pasteur Institute. Total grant: 541.077,00 € Lab subcontracting fee: 7.500,00 €
Operational Programme Competitiveness, Entrepreneurship and Innovation 2014-2020 (EPAnEK); sectoral operational programme of the Partnership and Cooperation Agreement (the new NSRF) for the period 2014-2020, approved on 18/12/2014 by the European Commission. MIS 5030605 (Proposal no: Τ1ΕΔΚ-03939, 13-06-2017), Research field: AGRI-FOOD, Titre: Free range pastured Poultry biochemical indicators (Novel and Dynamic) to certify Outdoor rearing Systems”. Acronym: P.I.N.D.O.S. Collaborating Institutions: 1. Biochemistry Laboratory, Dept of Chemistry, University of Ioannina, 2. Agricultural Poultry Association of Ioannina (APSI PINDOS) and 3. Immunology Laboratory, Dept of Immunology, Hellenic Pasteur Institute. Total budget: 999.486,26 €, Laboratory amount of grant: 190.000,00 €
2018-2020
NSRF, IPIROS Prefecture, MIS 5033100, 28/11/2017, Field: AGRI-FOOD. Title: Development of a model unit of industrial poultry rearing/breeding/farming. (IPIROS) 1. University of Ioannina 2. Agricultural, Poultry Farm Cooperative of Ioannina “PINDOS” 3. “AGROZOI” Public Limited Company- Industrial and Commercial Company-Agricultural and Poultry Farming Businesses 4. Immunology Laboratory, Immunology Department, Hellenic Pasteur Institute, Athens, Greece. Total budget: 466.000,00 €, Immunology Lab amount of grant: 24.800,00 €
2017-2020
institutional and collaborative projects
Institutional project entitled «Infectious, autoimmune and neurodegenerative diseases: study of the pathogenetic mechanisms and development of diagnostic, prognostic and therapeutic approaches» (MIS 5002486) implemented under the Action “Strategic Development on the Research and Technological Sector” funded by the Operational Program «Competitiveness, Entrepreneurship & Innovation» (NSFR 2014-2020), and co-financed by the Greek State & the European Regional Development Fund (550.000€), for the period Oct.2017–Oct.2019. KRIPIS II action- Co-ordinated by the Hellenic Pasteur Institute (Dr E. Karagouni). (P.Lymberi participant: Responsible for sub-project WP4.4: “Development of human monoclonal IgG autoantibodies able to penetrate living cells, for intracellular targeting of the nucleus or the cytoplasm”). Lab amount of grant: 22.487,17 €
HPI FUNDING
2021-2022
Hellenic Pasteur Institute (Decision of the Administration Council, Reference Number: 14365 / 28.12.2020) Project: Development of Serological immunoassays to get insight into the anti-SARS-CoV-2 immune response in Greece. In collaboration with the HPI Labs: Molecular Biology & Immunobiotechnology and Public Health-Diagnostics, and the Institut Pasteur Paris (provided us the respective plasmids for antigen expression at the Inspired NODE-HPI). Project Coordinator: Dr P. LYMBERI Total amount of grant: 21.000,00 €, Immunology Laboratory grant: 9.000,00 €
PRIVATE FUNDING
2018-2019
Donation by I. Kabouris for cancer research: Development of human monoclonal autoantibodies for targeting of cancer cells (P.L.: Coordinator). Total donation: 50.000,00€. Immunology Laboratory grant: 12.5000,00 €
- In our Laboratory
Supervision of undergraduate, MSc and PhD students [2012 – present: Total 25]
Internship Programs (2-6 months) for undergraduate students in Greek Universities and 1st or 2nd year students in foreign Universities [2012 – present: 15]
- In MSc Post-graduate Programs
National and Kapodistrian University of Athens:
in two Interdepartmental MSc Programs: “Clinical Biochemistry & Molecular Diagnosis”, and “Applications of Biology in Medicine “[1999 – present]
Course titles: “Natural and Pathological autoimmunity” (theory) and “Immunodiagnostic methodology used in thyroid autoimmune diseases: as an example of organ-specific autoimmune diseases” (practice)
Democritus University of Thrace:
MSc Program “Infectious Diseases-International Medicine: FROM BENCH TO BEDSIDE”. [2021]
Course titles: «Natural Autoantibodies” and “ELISA: general principles and applications to COVID-19”
School of Medicine, University of Thessaly:
MSc Program “Clinical Applications of Molecular Medicine”. [2018]
Course title: «The know-thyself of the Immune System: Its significance and applications in clinical practice”
- At the Annual Seminars of the Hellenic Society for Immunology
Seminar “Autoimmunity: From Molecular Biology to Clinical Practice” [2023]
Course title: "Natural antibodies: main characteristics and biological role"
- At the High School “Lycée Franco-Hellénique Eugène Delacroix”
Participation in Career Issues (“Forum des Métiers”/Jobs forum): One-day event organized annually by the LFH-ED (The French international school in Athens): Presentation of the professions of biologist, biochemist, chemist with an emphasis on that of the researchers and open discussion with the students). [14-02-2020, 15-02-2019, 23-02-2018]
Distribution of knowledge – Society
Alumni of Arsakeion High School, Athens, Greece. Conferences on COVID-19 pandemic, SARS-CoV-2 molecular characteristics and the concept of mRNA vaccines. [2021, 2022]
Association of Friends of the Hellenic Pasteur Institute: Invited speaker
Topic: “Pathological autoimmunity and Autoimmune Diseases. Does Physiological autoimmunity also exist?” The audience: Members and friends of the Association of pensioners of Olympic airlines Company staff (SS EAPAE) [January 2020]
Topic: “The know thyself of the immune system: its importance and applications in clinical practice”. Theocharakis Foundation, Athens, Greece [April 2017]
Thessaloniki’s International Fair (TIF): participation in the 82nd to the 87th TIF. Contributed to the exhibition stand of the Hellenic Pasteur Institute with an interactive board game for elementary school students based on knowledge about vaccines and their importance. (This game is presented and offered in every activity of our Institute, which is targeted at the general public). [2018-2023]
Inaugural event in the context of the 100-year celebration of HPI: Organization and participation in a scientific event in honor of the internationally renowned immunology Professor, Stratis Avrameas, held at the HPI Auditorium (funded by the HPI and the Association “Friends of HPI”) - under the auspices of the French Embassy in Athens [31-05-2019]
Award of the Hellenic Society of Immunology (2022) for the best study presented orally during the 12th Hellenic Congress of Immunology, entitled: “Study of human natural IgG-monoclonal antibodies with cell-penetrating ability, as intracellular vehicles of gold nanoparticles carrying paclitaxel, to MDA-MB-231 breast cancer cells”.
Award of the Hellenic Society of Immunology (2019) for the best study presented in poster during the 11th Hellenic Congress in Immunology, entitled: “Human monoclonal, cell-penetrating antibodies, derived from Multiple Myeloma patients’ sera, inhibit cellular motility and induce apoptosis of metastatic breast cancer cells”
Award of the Hellenic Society of Immunology (2010) for the best study presented orally during the 8th Hellenic Congress in Immunology, entitled: “Experimental Autoimmune Thyroiditis: Study of the minimal T-cell epitopes of a pathogenic peptide of human thyroglobulin in genetically susceptible and resistant mouse strains”
Award of the European Society for Reproductive Immunology (2009) for the best study presented orally during the 7th European Congress on Reproductive Immunology, entitled: “Suppressive effect of an embryo-derived factor to pathogenic T-cells causing thyroid autoimmunity”
General Secretary of Research and Technology, Evaluation of HPI research laboratories (2005) : Project of excellence entitled: “Autoantibodies able to penetrate living cells”
2023
Iatrou A, Gounari M, Sofou E, Zaragoza-Infante L, Markopoulos I, Sarrigeorgiou I, Petrakis G, Pechlivanis N, Roumeliotou-Dimou M, Panayiotidis P, Stamatopoulos B, Gkanidou M, Sandaltzopoulos R, Degano M, Koletsa T, Lymberi P, Psomopoulos F, Ghia P, Agathangelidis A, Chatzidimitriou A, Stamatopoulos K. N-Glycosylation of the Ig Receptors Shapes the Antigen Reactivity in Chronic Lymphocytic Leukemia Subset #201. J Immunol. 2023 Sep 1;211(5):743-754. doi: 10.4049/jimmunol.2300330
Rouka E, Jagirdar RM, Sarrigeorgiou I, Pitaraki E, Sinis SI, Varsamas C, Papazoglou ED, Kotsiou OS, Lymberi P, Giannou A, Hatzoglou C, Gourgoulianis KI, Zarogiannis SG. Changes in expression of mesothelial BBS genes in 2D and 3D after lithium chloride and ammonium sulphate induction of primary cilium disturbance: a pilot study. Pharmacol Rep. 2023 Oct;75(5):1230-1239. doi: 10.1007/s43440-023-00513-0
Tsitsami E, Sarrigeorgiou I, Tsinti M, Rouka EC, Zarogiannis SG, Lymberi P. Natural autoimmunity in oligoarticular juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2023 May 3;21(1):44. doi: 10.1186/s12969-023-00823-w
Sarrigeorgiou I, Stivarou T, Tsinti G, Patsias A, Fotou E, Moulasioti V, Kyriakou D, Tellis C, Papadami M, Moussis V, Tsiouris V, Tsikaris V, Tsoukatos D, Lymberi P. Levels of Circulating IgM and IgY Natural Antibodies in Broiler Chicks: Association with Genotype and Farming Systems. Biology (Basel). 2023 Feb 14;12(2):304. doi: 10.3390/biology12020304
Stivarou T, Papaioannou Ligeri, Sarrigeorgiou I, Avgoustakis K, Lymberi P., Human monoclonal natural IgG antibodies can penetrate MDA-MB-231 cells and transport intracellularly paclitaxel-loaded gold nanorods. J Drug Deliv Sci Technol. 2023 Feb 80:104109. doi: 10.1016/j.jddst.2022.104109
2022
Vasileiou C, Kalantzi S, Vachlioti E, Athanassopoulos CM, Koutsakis C, Piperigkou Z, Karamanos N, Stivarou T, Lymberi P, Avgoustakis K, Papaioannou D. New analogues of polyamine toxins from spiders and wasps: Liquid phase fragment synthesis and evaluation of antiproliferative activity. Molecules 2022, 27(2), 447; https://doi.org/10.3390/molecules27020447
Sarrigeorgiou I, Moschandreou D, Dimitriadis A, Tsinti G, Sotiropoulou E, Ntoukaki E, Eliadis P, Backovic M, Labropoulou S, Escriou N, Pouliakis A, Giannopoulou G, Gaitanarou E, Lazaridis K, Mentis A, Mamalaki A, Grouzi E, Lymberi P. Combined monitoring of IgG and IgA anti-Spike and anti-Receptor binding domain long term responses following BNT162b2 mRNA vaccination in Greek healthcare workers. PLoS One. 2022 Nov 21;17(11):e0277827. doi: 10.1371/journal.pone.0277827
2021
Lymberi P, Zannikou M, Hatzioannou A. (2021) Natural autoantibodies in health and disease. Reference Module in Biomedical Sciences, Elsevier, https://www.sciencedirect.com/science/article/pii/B9780128204726001973
Yilmaz V, Ulusoy C, Hajtovic S, Turkoglu R, Kurtuncu M, Tzartos J, Lazaridis K, Tuzun E. (2021) Effects of Teriflunomide on B Cell Subsets in MuSK-Induced Experimental Autoimmune Myasthenia Gravis and Multiple Sclerosis. Immunol Invest. 50:671-684. doi: 10.1080/08820139.2020.1785491.
2020
Sarrigeorgiou I, Kotsiou O, Rouka E, Perlepe G, Gourgoulianis K, Lymberi P, Zarogiannis S. (2020) Measurement of Natural antibodies (NAbs) against actin, DNA and trinitrophenol in the fluid of pleural effusion patients with various etiologies". European Respiratory Journal 56: 1137; DOI: 10.1183/13993003. congress-2020.1137. https://erj.ersjournals.com/content/56/suppl_64/1137
Koneczny I, Yilmaz V, Lazaridis K, Tzartos J, Lenz TL, Tzartos S, Tüzün E, Leypoldt F. (2020) Common Denominators in the Immunobiology of IgG4 Autoimmune Diseases: What Do Glomerulonephritis, Pemphigus Vulgaris, Myasthenia Gravis, Thrombotic Thrombocytopenic Purpura and Autoimmune Encephalitis Have in Common? Front Immunol. 11:605214. doi: 10.3389/fimmu.2020.605214.
Lazaridis K, Tzartos SJ. (2020) Myasthenia Gravis: Autoantibody Specificities and Their Role in MG Management. Front Neurol. 11 :596981. doi: 10.3389/fneur.2020. 596981.
Lazaridis K, Baltatzidi V, Tzartos S. (2020) Immunoadsorption of MuSK autoantibodies as a specific treatment of MuSK-induced experimental autoimmune myasthenia gravis. J Neuroimmunol. 339:577136. doi: 10.1016/j.jneuroim.2019. 577136.
Lazaridis K, Tzartos SJ. (2020) Autoantibody Specificities in Myasthenia Gravis; Implications for Improved Diagnostics and Therapeutics. Front Immunol. 11:212. doi: 10.3389/ fimmu.2020.00212.
2019
Ntoufa S, Gounari M, Papakonstantinou N, Binou D, Tyritidis I, Stivarou Th, Sarrigeorgiou I, Iatrou A, Stavroyianni N Anagnostopoulos A, Lymberi P, Stamatopoulos K. (2019) Detailed Functional Characterization of Splenic Marginal Zone Lymphoma: Uncovering Links between the Epigenetic and the Signaling Machinery. Blood 134 (Supplement_1):1512. https://doi.org/10.1182/ blood-2019-127909
Gounari M, Iatrou A, Kotta K, Sarrigeorgiou I, Lymberi P, Chatzidimitriou A, Stamatopoulos K. (2019) Changes in N-Glycosylation Induced by Somatic Hypermutation Modulate the Antigen Reactivity of the Immunoglobulin Receptors in CLL Stereotyped Subset #201. Blood 134 (Supplement_1): 1733. https://doi.org/10.1182/blood-2019-127852
Koneczny I, Rennspiess D, Marcuse F, Dankerlui N, Abdul Hamid M, Mané-Damas M, Maessen J, Van Schil P, Saxena A, Zisimopoulou P, Lazaridis K, Woodhall M, Karagiorgou K, Tzartos J, Tzartos S, De Baets MH, Molenaar PC, Marx A, Zur Hausen A, Losen M, Martinez-Martinez P. (2019) Characterization of the thymus in Lrp4 myasthenia gravis: Four cases. Autoimmun Rev. 18:50-55. doi: 10.1016/j.autrev. 2018.07.011.
Hahtapornsawan S, Lazaridis K, Criado FJ, Torsello GF, Bisdas T, Austermann M, Donas KP. (2019) CTA Assessment of Midterm Morphological Changes to Chimney Grafts Used in the Treatment of Juxtarenal Aortic Aneurysms. J Endovasc Ther. 26:697-703. doi: 10.1177/1526602819861747.
2017
Lazaridis K, DalianoudisI, Baltatzidi V, Rougkou V, Tzartos S.J. (2017) Specific removal of autoantibodies by extracorporeal immunoadsorption ameliorates experimental autoimmune myasthenia gravis. J Neuroimmunol. 312:24-30. doi:10.1016/j.jneuroim.2017.09.001.
Saxena A, Stevens J, Cetin H, Koneczny I, Webster R, Lazaridis K, Tzartos S, Vrolix K, NogalesGadea G, Machiels B, Molenaar PC, Damoiseaux J, De Baets MH, Simon-Keller K, Marx A, Vincent A, Losen M, Martinez-Martinez P. (2017) Characterization of an anti-fetal AChR monoclonal antibody isolated from a myasthenia gravis patient. Sci Rep. 7:14426. doi: 10.1038/s41598-017-14350-8.
Lazaridis K, Baltatzidi V, Trakas N, Koutroumpi E, Karandreas N and Tzartos Socrates. (2017) Characterization of a reproducible rat EAMG model induced with various human acetylcholine receptor domains. J. Neuroimmunol. 303:13-21. doi: 10.1016/j.jneuroim.2016.12.011.
Koneczny I, Stevens J, de Rosa A, Huda S, Huijbers M, Saxena A, Maestri M, Lazaridis K, Zisimopoulou P, Tzartos S, Verschuuren J, van der Maarel S, van Damme P, Vincent A, de Baets M, Molenaar P, Ricciardi R, Martinez-Martinez P, Losen M. (2017) IgG4 autoantibodies against muscle-specific kinase undergo Fab-arm exchange in myasthenia gravis patients. J. Autoimmun. 77:104-115 doi: 10.1016/j.jaut.2016.11. 005.
2016
ZANNIKOU M, BELLOU S, ELIADES P, HATZIOANNOU A, MANTZARIS M, CARAYANNIOTIS G, AVRAMEAS S, LYMBERI P. DNA-histone complexes as ligands amplify cell penetration and nuclear targeting of anti-DNA antibodies via energy-independent mechanisms. Immunology 2016, 147: 73-81
Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. (2016) Myasthenia gravis-autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 12:259-68. doi: 10.1038/nrneurol.2016.44.
2015
SALI AD, KARAKASILIOTIS I, EVANGELIDOU M, AVRAMEAS S, LYMBERI P. Immunological evidence and regulatory potential for cell-penetrating antibodies in intravenous immunoglobulin. ClinTransl Immunology 2015; Volume:4 Issue:10 Pages: e42; doi: 10.1038/cti.2015.18
HATZIOANNOU A, KANISTRAS I, MANTZOU E, ANASTASIOU E, PEPPA M, SARANTOPOULOU V, LYMBERI P, ALEVISAKI M. A Effect of Advanced Glycation End Products on Human Thyroglobulin's Antigenicity as Identified by the Use of Sera from Patients with Hashimoto's Thyroiditis and Gestational Diabetes Mellitus. Int J Endocrinol 2015; 2015:849615. doi: 10.1155/2015/849615. Epub 2015 Jul 2.142(2):300-6
2014
KANISTRAS J, HATZIOANNOU A, LYMBERI P. A novel pathogenic peptide of thyroglobulin (aa 2208-2207) induces autoreactive T-cell and B-cell responses in both high and low responder mouse strains Immunology 2014; 142(2):300-6
2012 HATZIOANNOU A, ALEVIZAKI M, CARAYANNIOTIS G, LYMBERI P Fine epitope mapping within the pathogenic thyroglobulin peptide 2340-2359: minimal epitopes retaining antigenicity across various MHC haplotypes are not necessarily immunogenic Immunology 2012; 135: 245-253
VASILEIOUV, SARANDOPOULOU V, PHILIPPOU G, LYMBERI P, ET AL. Does the presence of thyroid antibodies affect the course and outcome of pregnancy in type 1 diabetic women? Diabetologia 2012; 55: S436-7
2011
MATHIOUDAKI K, SCORILAS A, ARDAVANIS A, LYMBERI P, TSIAMBAS E, DEVETZI M, APOSTOLAKI A, TALIERI M, Clinical evaluation of PRMT1 gene expression in breast cancer. Tumor Biology 2011; 32:575-582
LYMBERI P, TERPOS E, HATZIOANNOU A, ET AL. High frequency of monoclonal immunoglobulins exhibiting natural autoantibody-like activity in patients with multiple myeloma and Waldenstrommacroglobulinemia. Blood 2011; 118(21): 1248-1249
2009
HATZIOANNOU A, BOBOU G, LYMBERI P, BARNEA E. Suppressive effect of an embryo-derived factor to pathogenic T-cells causing thyroid autoimmunity. J ReprodImmunol 2009; 81(2): 141-141
2007
AVRAMEAS S, TERNYNCK T, TSONIS IA, LYMBERI P. Naturally occurring B-cell autoreactivity: a critical overview. J Autoimmun 2007; 29:213-218
LIVADITI O, GIAMARELLOS-BOURBOULIS EJ, KAKKAS I, KAPSIMALI V, LYMBERI P, PAPASTARIADES C, DOUZINAS EE. Grouping of patients with common variable immunodeficiency based on immunoglobulin biosynthesis: comparison with a classification system on CD4-naïve cells. Immunol.Lett 2007; 114:103-9
HATZIOANNOU A, LIAKATA E, KARRAS E, THRASYVOULIDES A, ALEVIZAKI M, LYMBERI P. Pathogenicity of a human thyroglobulin peptide (2340-2359) in mice with high or low genetic susceptibility to thyroiditis. Immunology. 2007; 122: 343-349
THRASYVOULIDES A, LIAKATA E, LYMBERI P. Spreading of antibody reactivity to non-thyroid antigens during experimental immunization with human thyroglobulin. ClinExpImmunol 2007; 147:120-127
2006
PHILIPPOU G, SARANDOPOULOU V, ALEVIZAKI M, LYMPERI P ET AL. Type 1 diabetic pregnant women may develop postpartum thyroiditis independently of the presence of anti-thyroid antibodies. Diabetologia 2006; 49: 571-572
2005
KARRAS E, YANG H, LYMBERI P, CHRISTADOSS P. Human thyroglobulin peptide p2340 induces autoimmune thyroiditis in HLA-DR3 transgenic mice. J Autoimmun 2005; 24: 291-6
DAI Y, ELIADES P, CARAYANNIOTIS K, MCCORMICK DJ, KONG Y-CM, MAGAFFA V, CORDOPATIS P, LYMBERI P, CARAYANNIOTIS G. Thyroxine-binding antibodies inhibit T cell recognition of a pathogenic thyroglobulin epitope. J Immunol 2005; 174: 3105-10
2004
THRASYVOULIDES A, LYMBERI P. Antibodies cross-reacting with thyroglobulin and thyroid peroxidase are induced by immunization of rabbits with an immunogenic thyroglobulin 20mer peptide. ClinExpImmunol 2004; 138: 423-9
BARRETT K, LIAKATA E, RAO PV, WATSON P, WEETMAN A, LYMBERI P, BANGA JP, CARAYANNIOTIS G. Induction of hyperthyroidism in mice by intradermal immunization with DNA encoding the thyrotropin receptor. ClinExpImmunol 2004; 136: 413-422
TSITSILONIS OE, THRASYVOULIDES A, BALAFAS A, VOUTSAS JF, PAPAMICHAIL M, LYMBERI P. Serological detection of hepatitis B viral infection by a panel of solid-phase enzyme-linked imunosorbent assays (ELISA). J Pharmaceut Biomed Analysis 2004; 34: 811-822
2003
LIAKATA E, PHILIPPOU G, LYMBERI P,CARAYANNIOTIS G.Assessment of the frequency of mutant (hprt-) T lymphocytes from peripheral blood of patients with Hashimoto’s thyroiditis. Thyroid 2003;13: 631-6
ZAMANOU A, SAMIOTAKI M, PANAYOTOU G, MARGARITIS L, LYMBERI P. Fine specificity and subclass of IgG anti-actin autoantibodies differ in health and disease. J Autoimmun 2003; 20: 333-344
THRASYVOULIDES A, LYMBERI P. Evidence for intramolecular B-cell epitope spreading during experi-mental immunization with animmunogenic thyroglobulin peptide.ClinExpImmunol 2003;132: 401-7
KARRAS E, CARAYANNIOTIS G, LYMBERI P. Induction of murine thyroiditis by a non-dominant Ek –restricted peptide of human thyroglobulin. Immunology 2003; 108: 556-561
2002
ZAMANOU A, TSIROGIANNI A, TERZOGLOU C, BALAFAS A, ECONOMIDOU I, LYMBERI P. Anti-smooth muscle antibodies (ASMA) and anti-cytoskeleton antibodies (ACTA) in liver diseases: A comparison of classical indirect immunofluorescence with ELISA. J Clin Lab Analysis 2002; 16(4):194-200
TSITSILONIS OE, STOEVA S, ECHNER H, MARGOMENOU L, BALAFAS A, TROY DJ, VOELTER W, PAPAMICHAIL M, LYMBERI P. A skeletal muscle troponin T ELISA based on the use of an antibody against the soluble troponin T (16-31) fragment. J Immunol Methods 2002; 268:141-8
2001
THRASYVOULIDES A, SAKARELLOS-DAITSIOTIS M, PHILIPPOU G, SOUVATZOGLOU A, SAKARELLOS C, LYMBERI P. B-cell autoepitopes on the acetylcholinesterase-homologous region of human thyroglobulin: association with Graves’disease and thyroid eye disease.Eur J Endocrinology 2001; 145(2): 119-127
2000
VOELTER W, STOEVA S, SCHICK M, ECHNER H, BECK A, LEHMAN R, MULLEN A.M, CASSERLY U, TROY D.J, TSITSILONIS O.E, LYMBERI P, BAXEVANIS C.N, PAPAMICHAIL M. Αnalytical tools for rapid, sensitive, quantitative identification of potential meat quality markers: application of immunoassays, HPLC and capillary electrophoresis. J PraktChem 2000; 342: 179-191
DAI Y, CARAYANNIOTIS KA, ELIADES P, LYMBERI P, ET AL. Blockade of T-cell recognition of a pathogenic thyroglobulin epitope by thyroxine-specific antibodies. Faseb J 2000; 14(6): A999-A999
1999
DAI Y, CARAYANNIOTIS K, ELIADES P, LYMBERI P, SEPHERD P, KONG Y-CM, CARAYANNIOTIS G. Enhancing or suppressive effects of antibodies on processing of a pathogenic T-cell epitope in thyroglobulin. J Immunol 1999; 162: 6987-6992
PUBLICATIONS IN GREEK SCIENTIFIC JOURNALS
AVRAMEAS S, LYMBERI P. The immune system from the perspective of natural autoimmunity. Immunity (Edition of the Hellenic Society of Immunology) 2014, 10, 2: 99-103.
LYMBERI P, PHILIPPOU G. Thyroid autoimmnity: autoantigens, autoantibodies, autoreactive T lymphocytes, pathogenicity. Αrchives of Hellenic Medicine 1999, 16: 337-351.




