
Research in the Laboratory of Molecular Genetics focuses on mechanisms of chronic inflammation and immune disease pathogenesis, as well as the interactions between the immune system and the central nervous system (CNS). Emphasis is placed on the translation of knowledge from experimental models to human disease with the aim of finding novel biomarkers and therapeutic approaches.
The Probert group investigates immune mechanisms that control inflammation, demyelination and remyelination of the central nervous system (CNS) in diseases such as multiple sclerosis (MS) (1). The overall objective of the research is to identify mechanisms that promote brain neuroprotection and repair and to identify new therapeutic targets to prevent the transition of MS from the relapsing-remitting phase (RRMS) that is considered primarily inflammatory, to the secondary progressive phase (PMS) that is thought to be mainly neurodegenerative and is resistant to current immunotherapies.
There is a long-standing interest in understanding the complex biology of TNF in the brain. Work in our lab and others has identified cells and molecular pathways that are crucial for the preservation and repair of the myelin sheath, and the protection of neurons by TNF under chronic inflammatory conditions (2–6). This knowledge has contributed decisively to the development of novel TNF inhibitors that selectively neutralize the deleterious proinflammatory effects of soluble TNF/TNF receptor 1 pathways while maintaining the beneficial effects of membrane TNF/TNF receptor 2 pathways as next generation TNF inhibitors for the treatment of inflammatory diseases including MS (7). With this background, the group works to gain a better understanding of how brain immune cells switch between beneficial and deleterious functions during chronic inflammation, and the effects that aging and cellular senescence have on these mechanisms. Current research also investigates how the immune system loses tolerance to the brain in diseases like MS, so that selective antigen-specific immunotherapies can be developed for treatment of CNS autoimmunity. Knowledge from basic research is translated into the human disease wherever possible in collaboration with clinical colleagues.

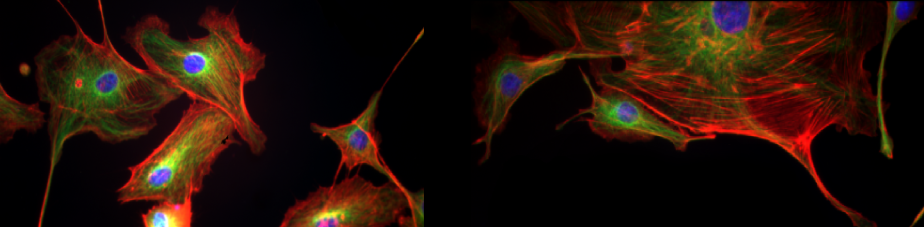
The TNF cytokine system in brain demyelination and remyelination
Remyelination is a spontaneous regenerative process of the adult CNS that is triggered in response to myelin damage (8). In MS, remyelination largely fails for unknown reasons, so that demyelinating lesions expand, and demyelinated neuronal axons are continuously exposed to injury by cytotoxic products of inflammation (9,10). We recently discovered a novel role for the proinflammatory cytokine soluble TNF as an inhibitor of remyelination in demyelinating brain lesions in an experimental model for PMS (cuprizone demyelination). Treatment of mice with XPro1595, a novel selective inhibitor of soluble TNF (11), not only reduced inflammation but also stimulated robust remyelination of demyelinated axons through the stimulation of microglia phagocytosis and clearance of myelin debris, thereby allowing oligodendrocyte precursor cells to produce new myelin and protect neurons (3,4). The molecular mechanisms that control the switching of CNS phagocytes such as microglia between beneficial and deleterious effects in the inflamed brain microenvironment are now under investigation so that additional targets for enabling CNS remyelination and repair can be identified, and therapeutic strategies for PMS can be developed. Also, the remyelination process itself is being investigated in more detail so that the beneficial responses of microglia and astrocytes that are stimulated by XPro1595 treatment, and their intercellular crosstalk, can be understood (This project is carried out in collaboration with Mr Raymond J. Tesi and Dr Christopher J. Barnum, CA, USA).
Members involved
Athena Boutou, Ilias Roufagalas, Katerina Politopoulou
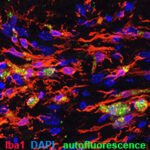
Aging and cellular senescence in MS pathology
Advancing age is the strongest predictor for the transition of MS from RRMS to PMS (12,13). The changes that are responsible for the development of irreversible disability are therefore likely to be age-related. We are studying the way in which increasing age affects lesion development in an experimental model of PMS (cuprizone demyelination) to identify cell types that may be prone to becoming senescent, and to understand the mechanisms by which ageing and cellular senescence might contribute to disease transition to the progressive form. We are also working to evaluate whether the inhibition of cellular senescence using brain-penetrating senomorphic drugs can rejuvenate the CNS as a potential approach for therapy in PMS.
Members involved
Irini Papazian, Maria Kourouvani, Dimitri Papadopoulos
Relevant publications
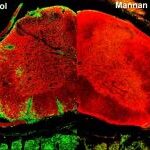
Antigen-specific therapies for autoimmunity
Autoimmune responses to the CNS are studied with the aim of finding new biomarkers in the blood and cerebrospinal fluid for monitoring the onset and progression of disease in MS patients, and novel experimental strategies that would specifically target the immune cells causing disease to be further developed as personalized immunotherapies in humans.
MS is an autoimmune disease of the CNS in which myelin-reactive T lymphocytes are thought to play a critical role. Current therapies inhibit disease in a rather non-specific way, either by globally suppressing immune responses or by preventing invasion of the CNS by immune cells. We have developed a novel approach that specifically inhibits the T lymphocytes and myeloid cells that cause disease in an experimental model for RRMS called experimental autoimmune encephalomyelitis (EAE) (14). Myelin peptides that are attached to the yeast polysaccharide mannan rapidly reverse inflammatory spinal cord lesions when injected into mice with ongoing disease. Protection involves the rapid alternative activation of immune system myeloid cells which prevents them from invading the CNS (15). Importantly, this approach specifically targets the autoimmune responses while preserving other immune responses and represents a promising approach for personalized immunotherapy in MS. (This project is carried out in collaboration with Professor Theodore Tselios, Dr Marilena Androutsou and Dr. Mary Anagnostouli).
Members involved
Anastasia Dagkonaki, Athina Papalambrou
Relevant publications

Genome-wide mapping of intracellular and extracellular noncoding RNAs from MS patient biofluids and their association with stress pathways
To gain a further understanding of MS at the genetic and epigenetic level, this project involves the transcriptomics analysis of blood and CSF samples of well characterized MS patients. The presence of various non-coding RNA (ncRNA) species will be analyzed and correlated to clinical phenotypes, disrupted stress-related pathways, as well as immune- and neuronal-specific networks. Such analysis will provide great insight into the genetic and epigenetic factors that might trigger the disease. On account of this, a database will be created in order to infer knowledge-based networks of differentially expressed RNA species using bioinformatics tools and methods already developed in the laboratory. The expression levels of selected ncRNA drivers of the above networks will be examined amongst the cargo of exosomes circulating in plasma and CSF. The results of comparative bioinformatics analysis of the blood and CSF transcriptome, combined with exosomics, is expected to unravel the ncRNA networks that are regulated by the interplay of the environment with the innate immune system and the CNS, as well as the ncRNA entities that are capable of effectively crossing the blood-brain barrier, leading to inflammation. The above research is expected to contribute to the development of innovative biomarkers for MS, but also to a more reasonable selection (based on real-time monitoring) of the immunomodulatory therapies that are currently being used in the clinical practice (This project is supervised by Dr. Antonis Giannakakis in collaboration with Dr. Maria Anagnostouli).
Members involved
Vasileios Gouzouasis, Antonis Giannakakis
Relevant publications
Our team seeks to understand the role of microglia in neuro-immunological conditions. Using MS animal models as our primary disease model system we employ a combination of live two-photon imaging, molecular, immunological and computational imaging analysis approaches (16–18).
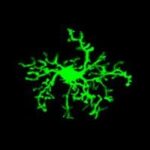
Microglia-driven pathology and altered brain surveillance in demyelination and neuroinflammation
Microglia are brain parenchyma-resident macrophages with various functions in health and disease. They are highly motile cells that constantly extend and retract their processes to perform brain surveillance, an homeostatic function by which they scan the tissue for possible infections, tissue damage and other pathological insults to resolve them. In pathology, they change their morphology and functions and they may have both beneficial and detrimental roles, depending on the disease context. We wish to understand how brain microglia respond to demyelinating insults and how their behaviour changes in recovery. To do so we developed a novel histopathological analysis approach in 3D and a cell-based analysis tool (https://github.com/VKyrargyri/MicroApp) that when applied in the cuprizone model revealed region- and disease- dependent changes in microglial dynamics in the brain grey matter during demyelination and remyelination (17). We now use similar approaches with the aim to unravel sensitive changes in microglial dynamics during neuroinflammation in the EAE model.
Members involved
Vasiliki Kyrgargyri, Ilias Roufagalas, Maria Avloniti, Maria Gomini
Relevant publications
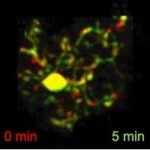
Brain resident microglia versus other tissue macrophages in MS models
The brain resident macrophages, which are the parenchymal microglia, the perivascular, meningeal and choroid plexus macrophages, have common cell origin with macrophages in the peripheral tissues. As a result, they share common molecular signatures making it challenging to differentiate their functions in neuroinflammatory diseases in which peripheral macrophages invade into the CNS. To deal with this, we employ constitutive knockout and tamoxifen-inducible gene-targeting approaches, immunological techniques, genetics and bioinformatics and currently seek to clarify the specific role of the brain resident microglial NF-κB molecular pathway versus other tissue macrophages in EAE.
Members involved
Vasiliki Kyragryri, Maria Avloniti, Maria Gomini, Adamantia Mitropoulou
Relevant publications
The Kambas group main focus is translational research with the principle from clinical bed to laboratory bench and back to the bed of the patient. The group aims to study mechanisms of disease under the lens of immunology and more specifically the innate immune system. Key focuses of the research are neutrophil and their functions, such as neutrophil extracellular traps, other innate immune populations and their contribution to diseases, hematopoiesis and more robust mechanisms that influence immune responses such as autophagy. The scientific interests branch in both infectious and non-infectious diseases.
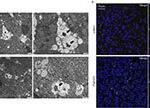
Neutrophils and NET
Neutrophils is a major research interest of this team. These cells are very understudied due to the stringent and unique conditions of handling they require that make their culturing conditions challenging. All protocols concerning human neutrophil handling and culturing have been established in HPI by Dr. Kambas and his team during his tenure. A fascinating mechanism of these cells that is unique to them in in vivo conditions is the generation of a neutrophil extracellular traps (NETs). Other cellular types demonstrate the capacity to execute this function but only under in vitro conditions. These chromatin structures are decorated by a plethora of variable proteins depending on the inflammatory environment, giving them the capacity to influence a variety of pathophysiological conditions (autoimmunity/autoinflammation, thrombosis, fibrosis, cancer). The team also studies the various mechanisms that are engaged for the generation of these structures, such as autophagy (19). Autophagy also constitutes a key immunoregulatory mechanism by regulating both innate and adaptive immune responses.
Moreover, it is one of the few teams worldwide that manage to generate in vitro fully functional granulocytes from hematopoietic stem cells (CD34) derived from bone marrow of human donors (20).
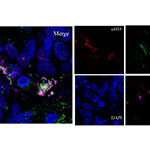
Innate immunity and infections
There is very little to almost no research done for the majority of microorganisms about their interaction with innate immune populations and most specifically with neutrophils, although neutrophils constitute the sentinels (first cells to arrive to an inflamed tissue) of immunity. The aim of the team is to try to bridge innate immune responses, such as neutrophil phagocytosis, degranulation and NET generation with various intracellular pathogens. The main focus of the team according to the current running projects are H. pylori, Legionella and Bartonella infections and how the immune system is implicated in the pathophysiology that originates from these parasites (gastritis/gastric metaplasia, lymphadenitis etc).
Members involved
Myrto Koutantou, Elena Petrou
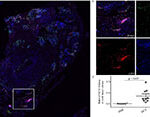
Innate immunity and non-infectious disorders
The group continues to investigate innate immune responses in sterile inflammation, following Dr. Kambas previous years of research in other groups. The main focus is neutrophil involvement in pathophysiology of autoimmune/autoinflammatory, thrombotic and fibrotic disorders. Currently the group is investigating the involvement of neutrophils and NETs in giant cell arteritis (21), antiphospholipid syndrome and rheumatoid arthritis. The team’s efforts are focused on identifying the key mechanisms that could be used as therapeutic targets in these conditions. To this end the group will branch out also in the study of anti-inflammatory lipid mediators (resolvins/proresolvins) in these disorders as targets against neutrophilic activation. Furthermore, the study group is expanding its interests to study senescence as an additional mechanism that could influence the inflammatory environment in these disorders.
Members involved
Stavros Naoum, Dimitrios Palamidas, Fotis Bantounas, Athanasios D. Bakasis
The team uses molecular and genetic tools such as Cre/loxP recombination in mice with experimental models of human disease, flow cytometry, microscopy, and most recently single cell RNA sequencing with bioinformatics tools, to study the cellular and molecular mechanisms of CNS pathology. The most-used experimental models are EAE, which models the autoimmune components of MS (22), and cuprizone-induced demyelination which is important for studying mechanisms of remyelination and recapitulates key aspects of PMS such as oxidative damage and mitochondrial injury in the presence of chronically activated microglia (23). In vivo and in vitro models for epilepsy and glutamate excitotoxicity are also available.
The laboratory maintains a repository of immune and disease mouse strains in the Transgenic Technology Unit (headed by Mr Fotis Badounas), and research is carried out in close collaboration with the Institute’s horizontal platforms, particularly the Department of Animal Models for Medical Research (add link to ΤΖΠΒΕ) and the Imaging Unit equipped with flow cytometry, confocal, time lapse and 2-photon imaging systems (add link to Imaging Unit), as well as expert external collaborators in areas of single cell RNA sequencing, bioinformatics, pharmaceutical development and clinical studies.
| 2020-2023 | Hellenic Foundation for Research and Innovation (HFRI) – Research Grant to support Faculty Members and Researchers entitled “Enhancing the beneficial functions of CNS macrophages to promote remyelination as a prototypic therapeutic strategy for the treatment of progressive multiple sclerosis”, acronym “MacRepair” (Ref: HFRI-FM17-2900). Principal Investigator L. Probert. Duration 36 months (28/02/2020-27/02/2023). Total budget 179.957,12 €. |
| 2020-2023 | Hellenic Ministry of Development and Investment, General Secretariat of Science and Innovation (GGEK) – Action for the Generation of a «National Network for Research into Genetic Neurodegenerative Diseases», acronym ΕΔΙΑΝ, under the action «National Infrastructure Research Networks in Areas of Precision Medicine and Climate Change» (Ref: 2018ΣΕ01300001). Duration 36 months (19/05/2020-18/05/2023). Total action budget 2.100.000,00 €; Hellenic Institute Pasteur participation coordinated by Rebecca Matsas, 150.000,00 €; Participant L. Probert 25.000,00 €. |
| 2019-2022 | Hellenic Academy of Neuroimmunology (HELANI) – Research Project entitled “Human multiple sclerosis CNS microenvironment immunoprofiling: Proof-of-principle for establishment of an integrated pipeline for biobank and analysis of MS samples” with acronym “MS Biobank”, Coordinators Lesley Probert & George Kollias, Principal Investigator L. Probert. Duration 40 months (09/2019-09/2022). Total budget 48.418,00 €. |
| 2018-2021 | Hellenic Foundation for Research and Innovation (HFRI) – Research Project to support Postdoctoral Researcher Dr. Vasiliki Kyrargyri for independent project entitled “Microglia-driven pathology and brain surveillance in demyelination’’ with acronym “MicroMS” (Action 1156). Duration 39 months (22/10/2018-21/01/2022). Total budget 180.000,00 €. |
| 2018-2021 | Greek General Secretariat of Science and Technology (GGET) – Research, Development & Innovation Project entitled “Development of an advanced humanized animal model for multiple sclerosis: application for pre-clinical study of new therapies”. Acronym “AKESO” (Ref: T1EDK01859). Coordinator M. Androutsou (Vianex SA), Scientific Coordinator and Principal Investigator L. Probert. Duration 36 months (06/09/2018 – 05/09/2022). Total budget 834.900,71 €, Laboratory of Molecular Genetics 397.680,71 €. |
| 2018-2019 | Multiple Sclerosis Society, UK – grant entitled “Is cellular senescence responsible for disability progression in progressive multiple sclerosis?”, (Ref: 75). Principal Investigator R Nicholas, Imperial College, London, UK. Participant L. Probert. Duration 9 months (14/05/2018-06/05/2019). Budget to team 40.000,00 UKP. |
| 2017-2019 | Greek General Secretariat of Science and Technology (GGET) – Institutional Project entitled “Infectious, autoimmune and neurodegenerative diseases: study of the pathogenic mechanisms and development of diagnostic, prognostic and therapeutic strategies” (Ref: MIS 5002486), acronym “KRIPIS II”. implemented under the Action “Strategic Development of the Research & Technology Sector” of the Operational Program “Competitiveness, Entrepreneurship and Innovation” (EPAnEK), and co-financed by the Hellenic State and the European Regional Development Fund. Duration 24 months (October 2017-October 2019). Co-ordinated by the Hellenic Pasteur Institute (Dr E. Karagkouni). Total Institute Budget 550,000.00, participant L. Probert, budget 25.000,00 €. |
| 2016-2019 | Multiple Sclerosis Trials Collaboration (MSTC), UK – grant entitled “The role of cellular aging in disability progression in multiple sclerosis”. Co-Principal Investigators L. Probert & D Papadopoulos. Duration 36 months (01/03/2016-01/03/2019). Budget to team 80.400,00 €. |
The laboratory is actively involved in communication and dissemination of neuroimmunology at national and international levels by participation in the following activities:
- European School of Neuroimmunology (ESNI) (www.esni.org).
- ESNI monthly Journal Club organized and chaired by Dr Maja Jagodic (Karolinska Institutet, SE) and Ms Stine Overdal (https://esni.isniweb.org/esni-journal-club-with-authors-tuesday-march-30-2021/).
- International Society of Neuroimmunology (ISNI) (www.isni.org).
- Hellenic Academy of Neuroimmunology (www.helani.gr).
2021
Boziki M, Styliadis C, Bakirtzis C, Grigoriadou E, Sintila AS, Nikolaidis I, Vrienniou A, Geys L, Pelidou SH, Probert L, Papazisis G, Bamidis P, Grigoriadis N. (2021). A National Representative, Cross-Sectional Study by the Hellenic Academy of NeuroImmunology (HEL.A.NI.) on COVID-19 and Multiple Sclerosis: Overall Impact and Willingness Toward Vaccination. Front. Neurol. 2021 Nov 25;12: 757038. doi: 10.3389/fneur.2021.757038. eCollection 2021.
Palamidas DA, Argyropoulou OD, Georgantzoglou N, Karatza E, Xingi E, Kapsogeorgou EK, Anagnostopoulos CD, Lazaris AC, Ritis K, Goules AV, Kambas K, Tzioufas AG. Neutrophil extracellular traps in giant cell arteritis biopsies: presentation, localization and co-expression with inflammatory cytokines. Rheumatology (Oxford). 2021 Jul 14:keab505.
Papazian I, Tsoukala E, Boutou A, Karamita M, Kambas K, Iliopoulou L, Fischer R, Kontermann RE, Denis MC, Kollias G, Lassmann H, Probert L. (2021). Fundamentally different roles of neuronal TNF receptors in CNS pathology: TNFR1 and IKKβ promote microglial responses and tissue injury in demyelination while TNFR2 protects against excitotoxicity in mice. J. Neuroinflamm. 2021 Sep 26; 18(1): 222. doi: 10.1186/s12974-021-02200-4.
Roufagalas I, Avloniti M, Fortosi A, Xingi E, Thomaidou D, Probert L, Kyrargyri V. (2021). Novel cell-based analysis reveals region-dependent changes in microglial dynamics in grey matter in a cuprizone model of demyelination. Neurobiol. Dis. 2021 Sep; 157: 105449. doi: 10.1016/j.nbd.2021.105449. Epub 2021 Jul 16.
Stergiou IE, Kambas K, Poulaki A, Giannouli S, Katsila T, Dimitrakopoulou A, Vidali V, Mouchtouris V, Kloukina I, Xingi E, Pagakis SN, Probert L, Patrinos GP, Ritis K, Tzioufas AG, Voulgarelis M. (2021). Exploiting the role of hypoxia-inducible factor 1 and pseudohypoxia in the myelodysplastic syndrome pathophysiology. Int. J. Mol. Sci. 2021 Apr 15;22(8): 4099. doi: 10.3390/ijms22084099.
2020
Daskalakis K, Alexandraki KI, Kloukina I, Kassi E, Felekouras E, Xingi E, Pagakis SN, Tsolakis AV, Andreakos E, Kaltsas G, Kambas K. Increased autophagy/mitophagy levels in primary tumours of patients with pancreatic neuroendocrine neoplasms. Endocrine. 2020 May;68(2):438-447.
Dagkonaki A, Avloniti M, Evangelidou M, Papazian I, Kanistras I, Tseveleki V, Lampros F, Tselios T, Jensen LT, Möbius W, Ruhwedel T, Androutsou ME, Matsoukas J, Anagnostouli M, Lassmann H, Probert L. (2020). Mannan-MOG35-55 reverses experimental autoimmune encephalomyelitis, inducing a peripheral type 2 myeloid response, reducing CNS inflammation, and preserving axons in spinal cord lesions. Front. Immunol. 2020 Nov 19;11: 575451. doi: 10.3389/fimmu.2020.575451. eCollection 2020.
Pegoretti V, Swanson KA, Bethea JR, Probert L, Eisel ULM, Fischer R (2020). Inflammation and oxidative stress in multiple sclerosis: consequences for therapy development. Oxid. Med. Cell Longev. 2020 May 12;2020: 7191080. doi: 10.1155/2020/7191080. eCollection 2020.
Probert L, Quintana FJ, Bar-Or A. (2020). Editorial: update on translational neuroimmunology - Research of ISNI 2018. Front. Immunol. 2020 Sep 30;11: 2012. doi: 10.3389/fimmu.2020.02012. eCollection 2020.
Kyrargyri, V., Madry, C., Rifat, A., Arancibia-Carcamo, I. L., Jones, S. P., Chan, V. T. T., … Attwell, D. (2020). P2Y (13) receptors regulate microglial morphology, surveillance, and resting levels of interleukin 1β release. Glia, 68(2), 328–344. https://doi.org/10.1002/glia.23719
2019
Kyrargyri V., Attwell D., Jolivet RB., Madry C. (2019). Analysis of signaling mechanisms regulating microglial process movement. Methods Mol Biol, 2034:191-205; doi: 10.1007/978-1-4939-9658-2_14.
2018
Papazian I, Voulgari-Kokota A, Kyrargyri V, Evangelidou M, Probert L. (2018). Mesenchymal stem cell protection of neurons against glutamate excitotoxicity involves reduction of NMDA-triggered calcium responses and surface GluR1 and is partly mediated by TNF. Int. J. Mol. Sci. 2018, 19, 651; doi:10.3390/ijms19020651.
2017
Karamita M, Barnum C, Mobius W, Tansey MG, Szymkowski DE, Lassmann H, Probert L. (2017). Therapeutic inhibition of soluble brain TNF promotes remyelination by increasing myelin phagocytosis by microglia. J. Clin. Invest. Insight, Apr 20; 2(8). pii: 87455. doi: 10.1172/jci.insight.87455. [Epub ahead of print].
2015
Probert L. (2015). TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience Aug 27:302:2-22. doi: 10.1016/ j.neuroscience. 2015.06.038. Epub 2015 Jun 24. Review.
Tseveleki V, Tselios T, Kanistras I, Koutsoni O, Karamita M, Vamvakas SS, Apostolopoulos V, Dotsika E, Matsoukas J, Lassmann H, Probert L. (2015). Mannan-conjugated myelin peptides prime non-pathogenic Th1 and Th17 cells and ameliorate experimental autoimmune encephalomyelitis. Exp. Neurol. 267:254-67; doi: 10.1016/j.expneurol.2014.10.019. Epub 2014 Oct 30.
Kyrargyri V, Vega-Flores G, Gruart A, Delgado-Garcia JM, Probert L. (2015). Differential contributions of microglia and neuronal IKKβ to synaptic plasticity and associative learning in alert behaving mice. Glia Apr; 63 (4): 549-566; doi: 10.1002/glia.22756. Epub 2014 Oct 9.
Evangelidou M, Karamita M, Vamvakas SS, Szymkowski DE, Probert L. (2015). Altered expression of oligodendrocyte and neuronal marker genes predicts the clinical onset of autoimmune encephalomyelitis and indicates the effectiveness of multiple sclerosis-directed therapeutics. J. Immunol. May ;192(9):4122-33. doi: 10.4049/jimmunol.1300633. Epub 2014 Mar 28.






