The Laboratory of Molecular Biology and Immunobiotechnology (LMBI) focuses on: a) the development of new biotechnological drugs (production of recombinant biomolecules such as antigens, antibodies and peptides of known and novel molecular targets and b) functional studies of molecular targets and biomolecules for the diagnosis and therapeutic applications of human disease.
Research Priorities:
– Recombinant antibodies-A powerful technique for immunotherapy
– Hepcidin: The master regulator of Iron homeostasis.
– Development immunoassays for human diseases.
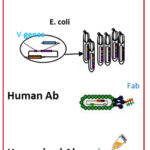
Recombinant phage display antibodies – A powerful technique for immunotherapy
Antibody phage display is an in vitro technology based on genetic engineering of bacteriophages in order to isolate high affinity antibody fragments after repeated rounds of antigen-driven selection and phage propagation. The LMBI is the pioneering lab in Greece where the phage antibody technology has been applied with great success, as well as the chain shuffling technology for in vitro affinity maturation of antibody fragments. One of the major advantages of expressing genetically engineered antibodies is that non-immunoglobulin sequences can be joined to antibody sequences creating multi-functional chimeric antibodies with a combining specificity that may be useful in targeting therapy.
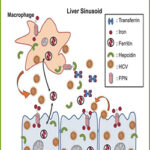
Hepcidin: The master regulator of Iron homeostasis:
The master regulator of systemic iron homeostasis is the liver peptide hepcidin (25-aa cysteine-rich antimicrobial peptide), which controls serum iron through degradation of ferroportin in iron-absorptive enterocytes and iron-recycling macrophages. Hepcidin is up-regulated by BMP/SMAD-signalling pathway under iron-replete hepatic conditions and by IL-6/STAT3 under inflammation and infection, and down-regulated by anaemia and hypoxia.
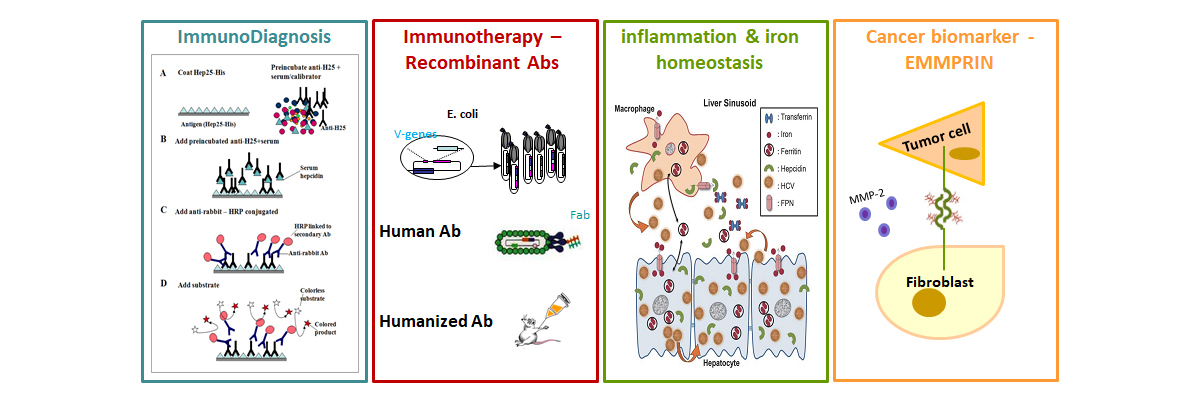
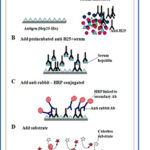
– Improving the diagnosis of iron disorders, through hepcidin quantification. Hepcidin is an important and promising biomarker that can complement already available markers for 1) diagnosis of genetic or acquired iron disorders, 2) clinical monitoring of patients with iron disorders and 3) personalized therapeutic treatment, especially in relation to safe iron supplementation. We have previously developed a competition Elisa assay for hepcidin quantification in human sera, based on a polyclonal antibody against the tagged recombinant peptide Hep25-His expressed in P.pastoris. The 2nd generation assay by using specific mAbs is under investigation.
– Understanding the role of iron in human health and disease. Although in most bacterial infections and in some viral infections there is an increased concentration of hepcidin in the serum of patients and experimental animals, no corresponding increase was observed in other cases like HCV. In order to investigate further the interactions between HCV and hepcidin we have investigated, in collaboration with Mol. Virology Lab, the effect of core and non-structural NS5A proteins on HAMP gene regulation and the effect of iron in viral replication in HCV-infected hepatocytes co-cultured with macrophages. Finally, using 3D-co-culture models of hepatocytes, macrophages and enterocytes that are involved in iron homeostasis, we investigated whether HCV alters innate immunity markers through HCV-imposed changes on intracellular iron levels that could favour viral persistence, thereby promoting liver disease progression.
– Evaluation of hepcidin agonist or antagonist administration as means to treat serious infections in an experimental animal model. Our previous work showed that following administration of mAbs in LPS-induced inflammation mice the hepcidin was suppressed. In this context, in collaboration with Bacteriology lab, the administration of hepcidin mAbs is under investigation for the treatment of serious bacterial infections.
Development of immunoassays
- the anti-SARS-CoV-2 immune response
This project is focused on the development of in-house highly specific and sensitive diagnostic ELISAs based on the use of the recombinant immunogenic proteins S (trimeric form), N, S1 and RBD, produced in the Expi293 rapid high-yield transient expression system (suspension-adapted HEK cells) and purified by affinity chromatography. Specific aim is to determine serological immune response after vaccination. A competitive ELISA is also developed to evaluate the presence of neutralizing antibodies that compete for human ACE2 binding and its validation is under investigation. This assay is of major importance, as it is useful for therapeutic plasma exchange, asymptomatic patient characterization and duration of the protective immunity.
- ANGPTLs
Angiopoietin-like proteins (ANGPTLs) are secreted lipid metabolism regulators that inhibit macrophagic/endothelial lipoprotein (LPL) and hepatic (HL) lipases, thereby increasing serum triglycerides. Additionally, ANGPTL-3 and -4 have both been involved in tumourigenesis. Specifically, ANGPTL-3 was found increased in liver biopsies of HCV- and HBV-induced hepatocellular carcinoma (HCC). ANGPTL-3 and ANGPTL-4 ELISA immunoassays were used to measure secreted protein levels in HCV-infected co-cultures of hepatοcytes/macrophages, where secreted ANGPTL-3 was decreased by at least 60% and ANGPTL-4 was up-regulated two-fold 4 days after infection. Collectively, HCV-induced modulation of ANGPTLs led to lipase activity changes that may alter intracellular and serum lipid levels during infection, thereby sustaining HCV replication and persistence in the lipid-poor environment of the chronically-infected liver. Ongoing studies with sera samples from healthy blood donors and patients with HCC, non-alcoholic steatohepatitis (NASH), acute or chronic HCV patients before and after antiviral treatment with Direct Acting Antivirals, showed differential regulation of ANGPTLs depending on liver disease stage. Therefore, the possibility that these proteins could serve as biomarkers for monitoring liver disease severity is currently under investigation.
“Hepcidin, the central regulator of iron homeostasis, as diagnostic biomarker and personalized therapeutic agent”, MIS 5032783, funded by the Operational Program «Competitiveness, Entrepreneurship and Innovation» (NSFR 2014-2020) of the GRST, and co-financed by the Greek State and the European Regional Development. (P.I.: A. Mamalaki)
“INSPIRED-HPI Node – The National Research Infrastructure on Integrated Structural Biology, Drug Screening Efforts and Drug target functional characterization, MIS 5002550, funded by the Operational Program «Competitiveness, Entrepreneurship and Innovation” (NSFR 2014-2020), and co-financed by the Greek State and the European Regional Development. (P.I.: A. Mamalaki)
“Initiation of large-scale production of SARS-CoV-2 antigens for serological immunoassays of SARS-CoV-2 antibodies and application in the upcoming national vaccination”. Research project funded by Hellenic Pasteur Institute 2021 (P.I.: P. Lymberi, A. Mamalaki, A. Mentis)
“Infectious, autoimmune and neurodegenerative diseases: study of the pathogenetic mechanisms and development of diagnostic, prognostic and therapeutic approaches” (MIS 5002486), funded by the Operational Program «Competitiveness, Entrepreneurship and Innovation» (NSFR 2014-2020), and co-financed by the Greek State and the European Regional Development Fund, for the period Oct. 2017 – Sept. 2019. (Participant, Coordinator: Dr Karagouni E)
1. Production of recombinant fragments of muscle acetylcholine receptor and their use for ex vivo immunoadsorption of anti-ACh receptor antibodies from myasthenic patients. Tzartos, S.J., Psaridi-Linardaki, L., Kostelidou, K. and Mamalaki. A. EP1509605 (B1)
2. Process for producing hepcidin A. Mamalaki, M. Marinou, V. Koliaraki US8614068 (B2)
3. Methods for Treating Neoplasia. K. Sidera, A. Mamalaki, L. Patsavoudi. US9328161 (B2) AU2011310664 (B2)
2021
Foka P, Dimitriadis A, Karamichali E, Kochlios E, Eliadis P, Valiakou V, Koskinas J, Mamalaki A, Georgopoulou U. 2022. HCV-Induced Immunometabolic Crosstalk in a Triple-Cell Co-Culture Model Capable of Simulating Systemic Iron Homeostasis. Cells. Aug 30;10(9):2251. (I.F. 6,6) doi: 10.3390/cells10092251
Dimitriadis A, Foka P, Kyratzopoulou E, Karamichali E, Petroulia S, Tsitoura P, Kakkanas A, Eliadis P, Georgopoulou U, Mamalaki A. 2020 The Hepatitis C virus NS5A and core proteins exert antagonistic effects on HAMP gene expression: the hidden interplay with the MTF-1/MRE pathway. FEBS Open Bio. Jan;11(1):237-250 (I.F. 2,693). doi: 10.1002/2211-5463.13048
Vaia Valiakou, Petros Eliadis, Eirini Karamichali, Ourania Tsitsilonis, John Koskinas, Urania Georgopoulou, and Pelagia Foka. 2021 Differential Expression of the Host Lipid Regulators ANGPTL-3 and ANGPTL-4 in HCV Infection and Treatment, Int J Mol Sci. Jul 26;22(15):7961. doi: 10.3390/ ijms22157961.
2018
Ivanova II, Mihaylova NM, Manoylov IK, Makatsori D, Lolov S, Nikolova MH, Mamalaki A, Prechl J, Tchorbanov AI. 2018 Targeting of Influenza Viral Epitopes to Antigen-Presenting Cells by Genetically Engineered Chimeric Molecules in a Humanized NOD SCID Gamma Transfer Model. Hum Gene Ther. Sep;29(9):1056-1070. (IF: 5.695) doi: 10.1089/hum.2018.100
Molyvdas A, Georgopoulou U, Lazaridis N, Hytiroglou P, Dimitriadis A, Foka P, Vassiliadis T, Loli G, Phillipidis A, Zebekakis P, Germenis AE, Speletas M, Germanidis G. 2018 The role of the NLRP3 inflammasome and the activation of IL-1β in the pathogenesis of chronic viral hepatic inflammation. Cytokine. Oct; 110: 389-396. doi: 10.1016/j.cyto.2018.04.032
2016
Foka P, Dimitriadis A, Karamichali E, Kyratzopoulou E, Giannimaras D, Koskinas J, Varaklioti A, Mamalaki A, Georgopoulou U. Alterations in the iron homeostasis network: A driving force for macrophage-mediated hepatitis C virus persistency. Virulence. 2016 Aug 17;7(6):679-90.
2015
Lyberopoulou A, Chachami G, Gatselis NK, Kyratzopoulou E, Saitis A, Gabeta S, Eliades P, Paraskeva E, Zachou K, Koukoulis GK, Mamalaki A, Dalekos GN, Simos G. Low Serum Hepcidin in Patients with Autoimmune Liver Diseases. PLoS One. 2015 Aug13;10(8):e0135486.
Kitsati N, Liakos D, Ermeidi E, Mantzaris MD, Vasakos S, Kyratzopoulou E, Eliadis P, Andrikos E, Kokkolou E, Sferopoulos G, Mamalaki A, Siamopoulos K, Galaris D. Rapid elevation of transferrin saturation and serum hepcidin concentration in hemodialysis patients after intravenous iron infusion. Haematologica. 2015 Mar;100(3):e80-3.
2014
Foka P, Dimitriadis A, Kyratzopoulou E, Giannimaras DA, Sarno S, Simos G, Georgopoulou U, Mamalaki A. A complex signaling network involving protein kinase CK2 is required for hepatitis C virus core protein-mediated modulation of the iron-regulatory hepcidin gene expression. Cell Mol Life Sci. 2014 Nov;71(21):4243-58.
Georgopoulou U, Dimitriadis A, Foka P, Karamichali E, Mamalaki A. Hepcidin and the iron enigma in HCV infection. Virulence. 2014 May 15;5(4):465-76.
2013
Papadimitropoulou A, Mamalaki A. The glycosylated IgII extracellular domain of EMMPRIN is implicated in the induction of MMP-2. Mol Cell Biochem. 2013 Jul;379(1-2):107-13.
2012
Rentoukas E, Tsarouhas K, Kaplanis I, Korou E, Nikolaou M, Marathonitis G, Kokkinou S, Haliassos A, Mamalaki A, Kouretas D, Tsitsimpikou C. Connection between telomerase activity in PBMC and markers of inflammation and endothelial dysfunction in patients with metabolic syndrome. PLoS One. 2012;7(4):e35739.
2011
Sidera K, El Hamidieh A, Mamalaki A, Patsavoudi E. The 4C5 cell-impermeable anti-HSP90 antibody with anti-cancer activity, is composed of a single light chain dimer. PLoS One. 2011;6(9):e23906.
Lioupis C, Barbatis C, Drougou A, Koliaraki V, Mamalaki A, Klonaris C, Georgopoulos S, Andrikopoulos V, Bastounis E. Association of haptoglobin genotype and common cardiovascular risk factors with the amount of iron in atherosclerotic carotid plaques. Atherosclerosis. 2011 May;216(1):131-8.
2010
Gattenlöhner S, Jörissen H, Huhn M, Vincent A, Beeson D, Tzartos S, Mamalaki A, Etschmann B, Muller-Hermelink HK, Koscielniak E, Barth S, Marx A. A human recombinant autoantibody-based immunotoxin specific for the fetal acetylcholine receptor inhibits rhabdomyosarcoma growth in vitro and in a murine transplantation model. J Biomed Biotechnol. 2010;2010:187621.
Tsochatzis E, Papatheodoridis GV, Koliaraki V, Hadziyannis E, Kafiri G, Manesis EK, Mamalaki A, Archimandritis AJ. Serum hepcidin levels are related to the severity of liver histological lesions in chronic hepatitis C. J Viral Hepat. 2010 Nov;17(11):800-6.
Lekka E, Gritzapis AD, Perez SA, Tsavaris N, Missitzis I, Mamalaki A, Papamichail M, Baxevanis CN. Identification and characterization of a HER-2/neu epitope as a potential target for cancer immunotherapy. Cancer Immunol Immunother. 2010 May;59(5):715-27.
2009
Kroot JJ, Kemna EH, Bansal SS, Busbridge M, Campostrini N, Girelli D, Hider RC, Koliaraki V, Mamalaki A, Olbina G, Tomosugi N, Tselepis C, Ward DG, Ganz T, Hendriks JC, Swinkels DW. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009 Dec;94(12):1748-52.
Dimitriadis A, Gontinou C, Baxevanis CN, Mamalaki A. The mannosylated extracellular domain of Her2/neu produced in P. pastoris induces protective antitumor immunity. BMC Cancer. 2009 Oct 30;9:386.
Koliaraki V, Marinou M, Vassilakopoulos TP, Vavourakis E, Tsochatzis E, Pangalis GA, Papatheodoridis G, Stamoulakatou A, Swinkels DW, Papanikolaou G, Mamalaki A. A novel immunological assay for hepcidin quantification in human serum. PLoS One. 2009;4(2):e4581.
2008
Koliaraki V, Marinou M, Samiotaki M, Panayotou G, Pantopoulos K, Mamalaki A. Iron regulatory and bactericidal properties of human recombinant hepcidin expressed in Pichia pastoris. Biochimie. 2008 May;90(5):726-35.
2006
Belimezi MM, Papanastassiou D, Merkouri E, Baxevanis CN, Mamalaki A. Growth inhibition of breast cancer cell lines overexpressing Her2/neu by a novel internalized fully human Fab antibody fragment. Cancer Immunol Immunother. 2006 Sep;55(9):1091-9
2005
Papadodima O, Sergaki M, Hurel C, Mamalaki A, Matsas R. Characterization of the BM88 promoter and identification of an 88 bp fragment sufficient to drive neurone-specific expression. J Neurochem. 2005 Oct;95(1):146-59.
Protopapadakis E, Kokla A, Tzartos SJ, Mamalaki A. Isolation and characterization of human anti-acetylcholine receptor monoclonal antibodies from transgenic mice expressing human immunoglobulin loci. Eur J Immunol. 2005 Jun;35(6):1960-8.
Psaridi-Linardaki L, Trakas N, Mamalaki A, Tzartos SJ. Specific immunoadsorption of the autoantibodies from myasthenic patients using the extracellular domain of the human muscle acetylcholine receptor alpha-subunit. Development of an antigen-specific therapeutic strategy. J Neuroimmunol. 2005 Feb;159(1-2):183-91
Fostieri E, Tzartos SJ, Berrih-Aknin S, Beeson D, Mamalaki A. Isolation of potent human Fab fragments against a novel highly immunogenic region on human muscle acetylcholine receptor which protect the receptor from myasthenic autoantibodies. Eur J Immunol. 2005 Feb;35(2):632-43.


